Evolution of Battery :
What is a battery?What are two basic types of batteries?
Two basic types of batteries: Primary(nonrechargeable battery where electricity stops when the chemicals are used up) and Secondary battery which can be recharged.

In 1799 Alessandro Volta(1745-1827),invented the “voltaic pile,”a pile of silver and zinc disks,separated by pieces of fabric saturated with sea water,that supplied an electric current when connected by a wire.
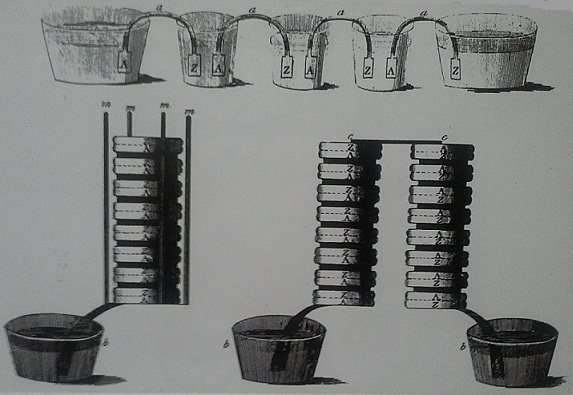
In voltaic pile,each cell had two electrodes- a positive one,the anode and a negative one,the cathode-suspended in a liquid known as the electrolyte.Later more efficient batteries were developed by inventors by implementing other combination of metals and electrolyte.In 1836 John F. Daniell an English chemist improves Volta’s cell by preventing the corrosion which is normally seen in Voltaic pile.
Gaston Plante, a French physicist developed a storage battery(secondary battery),which could be recharged and is similar to the battery that is used in today’s automobiles.In this secondary battery,lead plates were immersed in sulphuric acid. Gaston Plante produced this battery in 1859.


Gaston Plante(1834-1889),while working in Paris invented this lead-acid ancestor of today’s car battery.Lead-acid batteries remain the commonest type of vehicle battery to this day.In 1868 French chemist Georges Leclanche created the first “wet cell” battery also called as Leclanche cell.
In 1868 Dr. Carl Gassner invented a a variant of the Leclanché cell, which came to be known as the “dry cell” because it did not have liquid electrolyte and is dry.This dry cell is similar to today’s zinc-carbon battery which is manufactured still today.

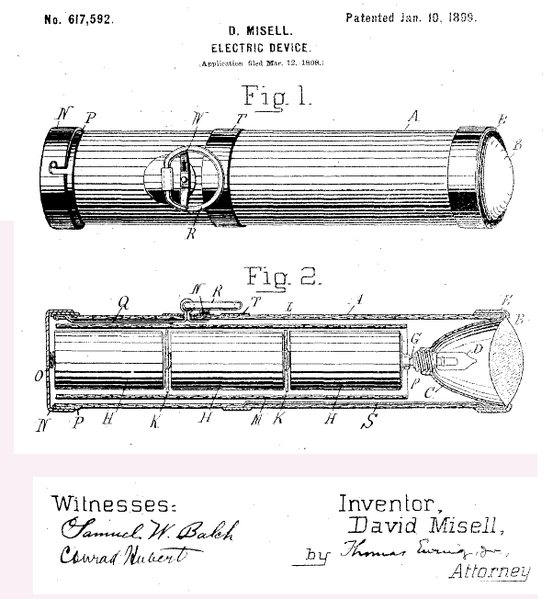
Thus after the invention of dry cells,solid electrolyte was used and the contents were encased in covers.Later in 1896 the first portable safe device known as the “Flashlight” was produced. The figure below is Misell’s Patent 617,592 line drawings showing cross section of flashlight with three cells, reflector and lens.
In 1900’s Thomas Alva Edison built a lighter and durable substitute to lead-acid battery which achieved great success in providing backup power for railroad crossing signals and lamps in mines.Though his model was good it did not outperform the lead-acid battery.The zinc-carbon batteries offered very low life and an employee from Union carbide developed alkaline battery in 1955 which offered longer battery life and these batteries hit market in 1959 but these were expensive.

These lithium ion batteries were commercially available from 1991 and these are the most powerful AA sized batteries available till date and are rechargeable.Experimentation with lithium batteries began in 1912 under G.N. Lewis, and in the 1970s the first lithium batteries were sold and Sony commercialized the lithium ion battery in 1991.A more efficient variant of this was made available from 1997 and is called as lithium ion polymer battery.
Future:From then the field of rechargeable batteries has evolved greatly by introducing products such as Nickel-Metal hydride batteries, titanium high-performance batteries, and 15-minute rechargeable batteries.In near future we will be using paper batteries which are flexible,ultra thin storage ormed by combining carbon nano tube with conventional cellulose based paper.For more details on paper battery visit this link-Click here
 Spinfold VisualDictionary-Evolutree- Technorip-Amazing Facts and much more.
Spinfold VisualDictionary-Evolutree- Technorip-Amazing Facts and much more.





